Electronegativity From Top to Bottom on the Periodic Table
The result of this change is that electronegativity increases from bottom to top in a column in the periodic table even though there are more protons in the elements at the bottom of the column. The electronegativity of atoms increases as you move from left to right across a period in the periodic table.

Electronegativity Definition Value Chart And Trend In Periodic Table
Electronegativity is the measure of attraction of an atom to form chemical bonds with other atoms.

. The trend in electronegativity can be seen by the graph given below for group 7. When we go down in a group in the periodic table electronegativity decreases. Moving down any group on the periodic table results in the gain of electrons that occupy.
Electronegativity DECREASES down a Group but INCREASES across a Period from left to right. By referring only to the periodic table select the most electronegative element in group 6A. As we move from top to bottom in periodic table the atomic sizes increasesThe electrons are added in next energy level in every next element.
An atom that gains an electron will form a positive ion true or false. By referring only to the periodic table select the least electronegative element in the group Al Si P. What scientist first arranged elements by atomic number.
Periodic Trends In Electronegativity Ck 12 Foundation The modern form of the periodic table follows the modern periodic law that the properties of the elements are the function of the atomic number. This was just a brief layout of the trends in electronegativity of an element in the periodic table. 5 degrees email protected.
When we exclude the Noble Gases elements to the right of the Periodic Table are the most electronegative cf. The higher the electronegativity of an atom the greater its ability to attract shared electrons. This is because as you go from left to right across a period the nuclear charge is increasing faster than the electron shielding so the attraction that the atoms have for the valence electrons increases.
As we go down from top to bottom on periodic table more electron shells are added also more protons are added. The electronegativity trend refers to a trend that can be seen across the periodic tableThis trend is seen as you move across the periodic table from left to right. From left to right.
The electronegativity of elements on the periodic table tends to increase _____ From left to right top to bottom From right to left. It is the ability of an atom to attract the shared electronsThe greater the ability of attraction higher will be the electronegativity. For example the electronegativity trend across period 3 in the periodic table is depicted below.
Electronegativity increases moving from bottom to top of the periodic table because the distance between the nucleus and outermost electrons decreases. The electronegativity increases while it decreases as you move down a group of elements. The electronegativity of atoms decreases as you move from top to bottom down a group in the periodic table.
The electronegativity of elements increases from left to right and from top to bottom on the periodic table true or false. It also increases from left to right as the increasing number of protons creates an increased nuclear charge. Electronegativity is conceived to be the ability of an atom in an element to polarize electron density towards itself.
As you move from left to right across the periodic table atoms have a greater nuclear charge and a. It may be noted that the elements belonging to the same group are said to constitute a family. Explore each Elements Electronegativity in one interactive Periodic Table.
Electronegativity _____ from left to right within a period and _____ from top to bottom within a group. As new shells are added ability of nucleus to. We review their content and use your feedback to keep the quality high.
As we move across a period from left to right the nuclear charge increases and the atomic size decreases therefore the value of electronegativity increases across a period in the modern periodic table. View the full answer. This list contains the 118 elements of chemistry.
Experts are tested by Chegg as specialists in their subject area. Electronegativity values generally increase from left to right across the periodic table. The electronegativity increases as you move bottom to top and left to right on the periodic table.
Click on any elements name for further chemical properties environmental data or health effects. The trend is shown below. Electronegativity is tendency of an atom to attract electrons.
Here fluorine has the highest electronegativity 40. Electronegativity Across a period from left to right the electronegativity of atoms increases. Elements at the top of a column have greater electronegativities than elements at the bottom of a given column.
Answer 1 of 7. Chemical elements listed by electronegativity The elements of the periodic table sorted by electronegativity. Electronegativities of nitrogen oxygen and.
Because you are moving towards Fluorine which is. The electronegativity increases as you move bottom to top and left to right on the periodic table. A similar rationale can explain why electronegativity decreases from top to bottom on the periodic table.
Explore all 118 elements and their Electronegativity numbers. Electronegativities generally decrease from top to bottom of a group. Electronegativity is a measure of the ability of an atom to attract the electrons when the atom is part of a compound.
Because you are moving towards Fluorine which is. While this is the basic definition of the electronegativity trend to truly understand it it would be helpful to. The result of this change is that electronegativity increases from bottom to top in a column in the periodic table even though there are more protons in the elements at the bottom of the column.
The highest electronegativity value is for fluorine. Periodic Trends in the Electronegativities of Elements. The electronegativity of atoms increases as you move from left to right across a period in the periodic table.
Elements at the top of a column have greater electronegativities.
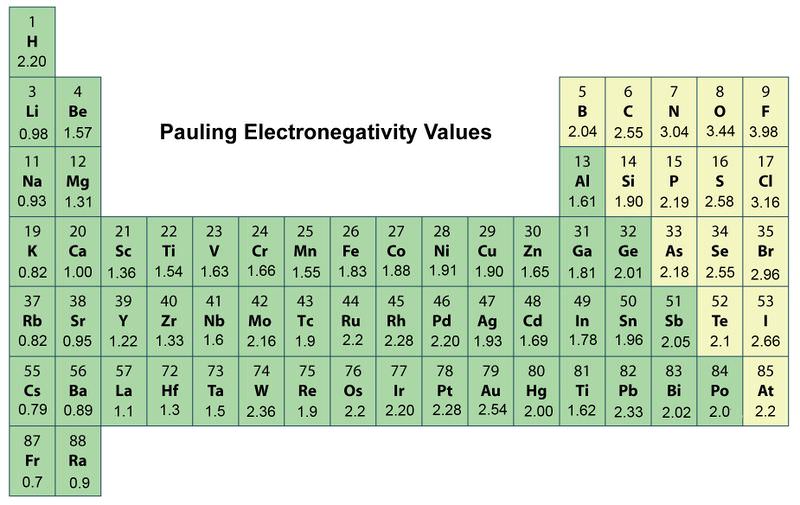
Periodic Trends In Electronegativity Ck 12 Foundation


No comments for "Electronegativity From Top to Bottom on the Periodic Table"
Post a Comment